Multiple Choice
Hydrogen sulfide ion, HS-, can react differently depending on the acidity of the solution in which it is present.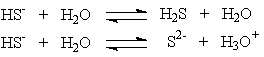 These two solutions show that the
These two solutions show that the
A) hydrogen sulfide ion is an acid.
B) hydrogen sulfide ion is a base.
C) hydrogen sulfide ion is amphoteric.
D) hydrogen sulfide ion can only react with water.
Correct Answer:

Verified
Correct Answer:
Verified
Q38: The carbonate/hydrogen carbonate buffer is responsible for
Q39: A sample of blood has a pH
Q40: Calculate the pH of a 0.0019 M
Q41: Calculate the [OH<sup>-</sup>] in an aqueous solution
Q42: The ethylammonium ion, CH<sub>3</sub>CH<sub>2</sub>NH<sub>3</sub><sup>+</sup> has a pK<sub>a</sub>
Q44: When we talk about pH we are
Q45: What is the [OH<sup>-</sup>] of a solution
Q46: In the reaction below:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="In the
Q47: A base is a compound that produces
Q48: Basic substances tend to have a _