Multiple Choice
In the reaction below: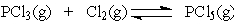 Increasing the concentration of Cl2 will ___ at equilibrium.
Increasing the concentration of Cl2 will ___ at equilibrium.
A) increase the concentration of PCl5
B) increase the concentration of PCl3
C) decrease the concentration of PCl5
D) have no effect
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Calculate the [OH<sup>-</sup>] in an aqueous solution
Q42: The ethylammonium ion, CH<sub>3</sub>CH<sub>2</sub>NH<sub>3</sub><sup>+</sup> has a pK<sub>a</sub>
Q43: Hydrogen sulfide ion, HS<sup>-</sup>, can react differently
Q44: When we talk about pH we are
Q45: What is the [OH<sup>-</sup>] of a solution
Q47: A base is a compound that produces
Q48: Basic substances tend to have a _
Q49: The conjugate acid of the hydrogen sulfate
Q50: The greater the hydronium ion concentration, the
Q51: Which of the following are the strong