Multiple Choice
The equilibrium constant, Keq, for the ammonia synthesis below is found to be 6.0 x 10-2 at 500 C. Which of the following statements is true at equilibrium? 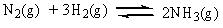
A) Product concentration is greater than reactant concentration.
B) Reactant concentration is greater than product concentration.
C) Reactant concentration is the same as product concentration.
D) Relative concentrations of reactants and products cannot be predicted.
Correct Answer:

Verified
Correct Answer:
Verified
Q81: What is the [H<sub>3</sub>O<sup>+</sup>] of a solution
Q82: K<sub>w</sub>, the equilibrium constant for the ionization
Q83: A K<sub>a</sub> can be calculated for some
Q84: The higher the numerical value of an
Q85: A is red, B is colorless, and
Q86: What volume of 0.200 M HCl is
Q87: When a reaction is at equilibrium,<br>A) it
Q88: Antacids may contain which ion to reduce
Q90: The carbonate/hydrogen carbonate buffer is responsible for
Q91: Which of the following is the correct