Multiple Choice
If 5.5 moles of pentane are burned according to the following equation: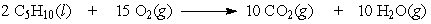 how many moles of oxygen are required?
how many moles of oxygen are required?
A) 41
B) 83
C) 11
D) 5.5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt=" " class="answers-bank-image d-block" rel="preload"
Q4: Methanol burns in air according to the
Q5: In the following reaction, how many grams
Q6: If there is a "limiting reactant" in
Q7: Benzoyl peroxide can be used as a
Q9: Platinum serves to catalyze some hydrogenation reactions.
Q10: According to the following equation, what mass
Q11: 2-methylcyclopentene reacts with hydrogen in the presence
Q12: While this isn't a real reaction, we
Q13: Balance the equation. Baking soda (NaHCO<sub>3</sub>) can