Essay
Balance the equation. Baking soda (NaHCO3) can be used as an antacid. It reacts with and neutralizes stomach acid (HCl). The equation for this reaction is as follows:
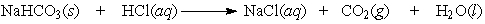
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: If 5.5 moles of pentane are burned
Q9: Platinum serves to catalyze some hydrogenation reactions.
Q10: According to the following equation, what mass
Q11: 2-methylcyclopentene reacts with hydrogen in the presence
Q12: While this isn't a real reaction, we
Q14: Compounds are formed from elements in a
Q15: Write and balance the equation for the
Q16: An oxidation reaction can be defined as
Q17: Sodium stearate is a soap that is
Q18: Aluminum carbonate was once the active ingredient