Short Answer
Aluminum carbonate was once the active ingredient in Rolaids . It reacts with stomach acid according to the following equation:
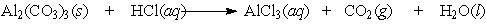 A single aluminum carbonate tablet weighs 2.48 g. How many grams of AlCl3 would be produced by the reaction of a single tablet?
A single aluminum carbonate tablet weighs 2.48 g. How many grams of AlCl3 would be produced by the reaction of a single tablet?
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Balance the equation. Baking soda (NaHCO<sub>3</sub>) can
Q14: Compounds are formed from elements in a
Q15: Write and balance the equation for the
Q16: An oxidation reaction can be defined as
Q17: Sodium stearate is a soap that is
Q19: A test used by geologists to determine
Q20: Tums tablets are composed of the substance
Q21: The coefficients used to balance chemical equations
Q22: In a hydrolysis reaction, water is used
Q23: In the reaction below: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="In