Multiple Choice
Using these metal ion/metal standard reduction potentials 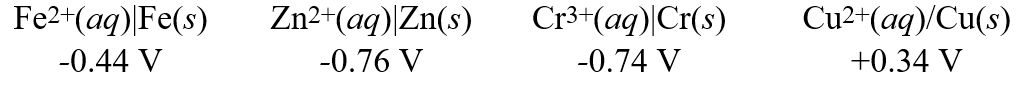 Calculate the standard free energy ( G°) change for the cell reaction:Fe2+(aq) + Cr(s) Fe(s) + Cr3+(aq)
Calculate the standard free energy ( G°) change for the cell reaction:Fe2+(aq) + Cr(s) Fe(s) + Cr3+(aq)
A) -92.6 kJ
B) -86.8 kJ
C) 683.1 kJ
D) -57.9 kJ
E) -173.7 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q95: Using the standard reduction potentials <img
Q96: Anions<br>A)are charged ions that move toward the
Q97: How long will it take to produce
Q98: Consider this electrochemical cell:Pt | Pu<sup>3+</sup>(aq),
Q99: A metal object is to be gold-plated
Q101: The electric charge can be calculated as<br>A)the
Q102: Sketch a galvanic cell with metallic zinc
Q103: One mole of electrical charge contains<br>A)4.184 joules.<br>B)3,600
Q104: The half-reaction that occurs at the
Q105: When an aqueous solution of magnesium sulfate