Multiple Choice
Using the standard reduction potentials 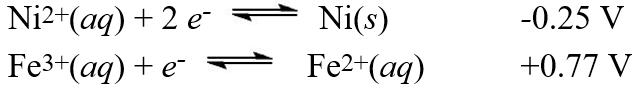 Calculate the value of E°cell for the cell with the following reaction.
Calculate the value of E°cell for the cell with the following reaction.
Ni2+(aq) + 2 Fe2+(aq) Ni(s) + 2 Fe3+(aq)
A) +0.52 V
B) -1.02 V
C) +2.81 V
D) +1.02 V
E) -2.81 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: The solubility product constant for NtCrO<sub>4</sub> is
Q91: The half-reaction that should occur at
Q92: The anode in a galvanic cell has
Q93: Consider these metal ion/metal standard reduction potentials
Q94: Using the reduction potentials given, calculate the
Q96: Anions<br>A)are charged ions that move toward the
Q97: How long will it take to produce
Q98: Consider this electrochemical cell:Pt | Pu<sup>3+</sup>(aq),
Q99: A metal object is to be gold-plated
Q100: Using these metal ion/metal standard reduction