Multiple Choice
Using the reduction potentials given, calculate the equilibrium constant, K, at 25°C for the reaction, 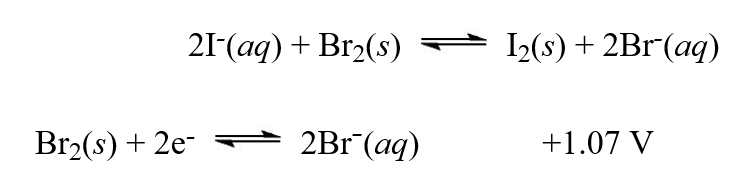
A) 8.5 × 1017
B) 6.8
C) 2.4 × 104
D) 1.02
E) 1.2 × 10-18
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q80: Using the same current and similar conditions,
Q81: If a brine bath is stirred during
Q82: Given the following notation for an electrochemical
Q83: How many coulombs of charge are required
Q84: How many coulombs of electrical charge must
Q86: The products of the electrolysis of aqueous
Q87: Using the same current and similar conditions,
Q88: A galvanic cell has two electrodes. Which
Q89: An electrolytic cell has two electrodes. Which
Q90: The solubility product constant for NtCrO<sub>4</sub> is