Short Answer
The solubility product constant for NtCrO4 is 4.50 × 10-12. Using the potentials provided in the listing above, calculate the value for the equilibrium constant, Kc, for the reaction Nt(CN)42-(aq)+ CrO42-(aq)  NtCrO4(s)+ 4CN-(aq)Use these standard potentials in answering this question. (Note that imaginary elements are being used in these questions).
NtCrO4(s)+ 4CN-(aq)Use these standard potentials in answering this question. (Note that imaginary elements are being used in these questions). 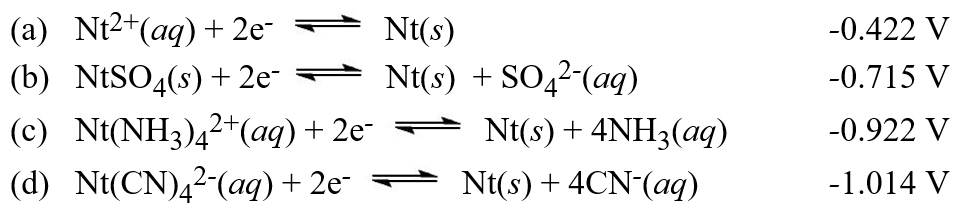 Hint: Combine two reactions in the list to find a third reaction and an equilibrium constant that combines with the solubilization of NtCrO4 to get the target equation.
Hint: Combine two reactions in the list to find a third reaction and an equilibrium constant that combines with the solubilization of NtCrO4 to get the target equation.
Correct Answer:

Verified
Correct Answer:
Verified
Q85: Using the reduction potentials given, calculate the
Q86: The products of the electrolysis of aqueous
Q87: Using the same current and similar conditions,
Q88: A galvanic cell has two electrodes. Which
Q89: An electrolytic cell has two electrodes. Which
Q91: The half-reaction that should occur at
Q92: The anode in a galvanic cell has
Q93: Consider these metal ion/metal standard reduction potentials
Q94: Using the reduction potentials given, calculate the
Q95: Using the standard reduction potentials <img