Multiple Choice
Wax is a solid mixture of hydrocarbon compounds consisting of molecules with long chains of carbon atoms. Which solvent would you expect to be most capable of dissolving wax?
A) 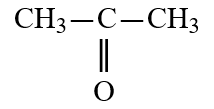
B) 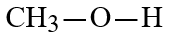
C) 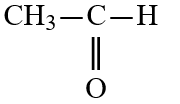
D) 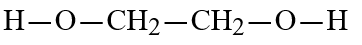
E) 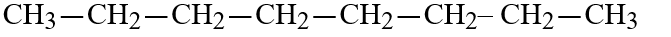
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: When a nonvolatile solute such as ammonium
Q3: Many marine organisms that require oxygen live
Q4: Dry air is a mixture of gases
Q5: Calculate the freezing point of a solution
Q6: Arrange these aqueous solutions in order of
Q7: Pure cyclohexane, C<sub>6</sub>H<sub>12</sub>, has a molar mass
Q8: Pure glacial acetic acid, HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>, has a
Q9: What is the mole fraction of ethylene
Q10: At 28.0°C, the vapor pressure of npropyl
Q11: An aqueous ethylene glycol solution being