Multiple Choice
Arrange these aqueous solutions in order of increasing boiling points: 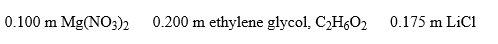
A) C2H6O2 < Mg(NO3) 2 < LiCl
B) Mg(NO3) 2 < LiCl < C2H6O2
C) C2H6O2 < LiCl < Mg(NO3) 2
D) LiCl < C2H6O2 < Mg(NO3) 2
E) Mg(NO3) 2 < C2H6O2 < LiCl
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: When a nonvolatile solute such as ammonium
Q2: Wax is a solid mixture of hydrocarbon
Q3: Many marine organisms that require oxygen live
Q4: Dry air is a mixture of gases
Q5: Calculate the freezing point of a solution
Q7: Pure cyclohexane, C<sub>6</sub>H<sub>12</sub>, has a molar mass
Q8: Pure glacial acetic acid, HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>, has a
Q9: What is the mole fraction of ethylene
Q10: At 28.0°C, the vapor pressure of npropyl
Q11: An aqueous ethylene glycol solution being