Multiple Choice
An aqueous solution of glycerol, C3H8O3, is 48.0% glycerol by mass and has a density of 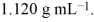 Calculate the molality of the glycerol solution.
Calculate the molality of the glycerol solution.
A) 11.2 m
B) 5.84 m
C) 0.584 m
D) 0.521 m
E) 10.0 m
Correct Answer:

Verified
Correct Answer:
Verified
Q43: An aqueous solution of glycerol, C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>, is
Q44: A solution of ethylene glycol (C<sub>2</sub>H<sub>6</sub>O<sub>2</sub>)in water
Q45: An aqueous solution that would cause red
Q46: If the concentration of hydrogen gas in
Q47: All substances can be either hydrophobic or
Q49: Pure cyclohexane, C<sub>6</sub>H<sub>12</sub>, has a freezing point
Q50: Which is a concentration unit whose value
Q51: What is the freezing point of an
Q52: Which aqueous solution has the highest boiling
Q53: A solution contains 221 g of glycerol