Multiple Choice
A solution of ethylene glycol (C2H6O2) in water is 3.981 molar and has a density of 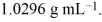 Calculate the percent, by weight, of ethylene glycol in the solution.
Calculate the percent, by weight, of ethylene glycol in the solution.
A) 3.867%
B) 4.099%
C) 15.14%
D) 24.00%
E) 25.45%
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Pure cyclohexane, C<sub>6</sub>H<sub>12</sub>, has a molar mass
Q40: Raoult's law expresses that the higher the
Q41: The solubility of O<sub>2</sub> in water is
Q42: What properties does a molecule need to
Q43: An aqueous solution of glycerol, C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>, is
Q45: An aqueous solution that would cause red
Q46: If the concentration of hydrogen gas in
Q47: All substances can be either hydrophobic or
Q48: An aqueous solution of glycerol, C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>, is
Q49: Pure cyclohexane, C<sub>6</sub>H<sub>12</sub>, has a freezing point