Multiple Choice
The following questions refer to the phase diagram below. 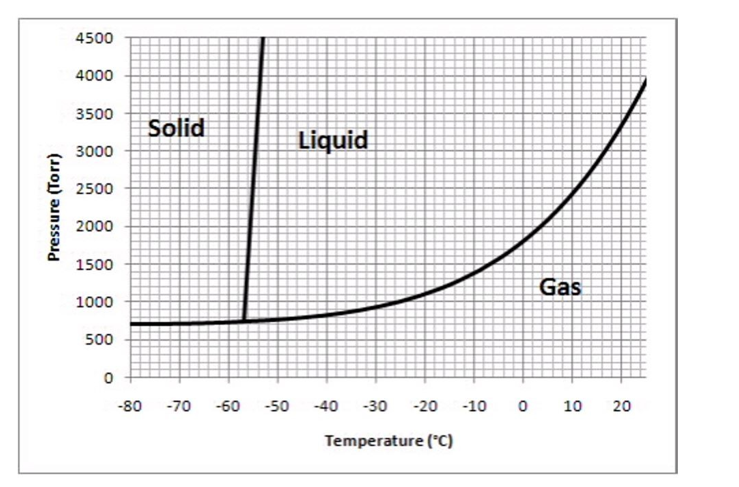
-Which of the following values of temperature and pressure most closely correspond to the triple point of this substance?
A) -21°C and 1000 torr
B) 0°C and 1000 torr.
C) -57°C and 740 torr
D) -50°C and 4500 torr
E) 0°C and 1760 torr
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Which one of the following substances is
Q11: Arrange these compounds in order of increasing
Q12: Given the following substances and their normal
Q13: At 1.0 atm pressure, ice (solid H<sub>2</sub>O)floats
Q14: An unknown solid is soft and lustrous.
Q16: Which covalent compound will exhibit hydrogen bonding
Q17: Solid carbon dioxide never forms a liquid
Q18: The following questions refer to the diagram
Q19: Which of the following liquids, at the
Q20: In a one-element solid with a simple