Short Answer
The following questions refer to the diagram below. 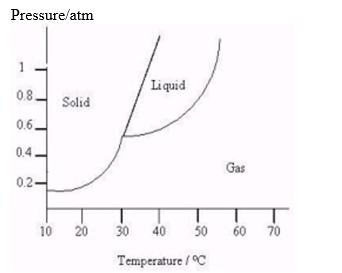
-How does the boiling point of this substance vary as the pressure on this substance increases?
Correct Answer:

Verified
As the pressure on t...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q13: At 1.0 atm pressure, ice (solid H<sub>2</sub>O)floats
Q14: An unknown solid is soft and lustrous.
Q15: The following questions refer to the phase
Q16: Which covalent compound will exhibit hydrogen bonding
Q17: Solid carbon dioxide never forms a liquid
Q19: Which of the following liquids, at the
Q20: In a one-element solid with a simple
Q21: An unknown solid is hard and brittle
Q22: Dioxane, C<sub>4</sub>H<sub>8</sub>O<sub>2</sub>, has an enthalpy of fusion
Q23: A unit cell of sodium chloride (face-centered