Multiple Choice
For the working galvanic cell shown at standard conditions, how would you increase the cell potential?(If needed, refer to Table 17-1 in the text ) 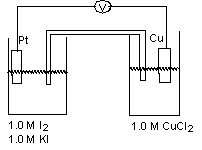
A) Make the Pt electrode larger.
B) Make the Copper electrode larger.
C) Increase the concentration of KI.
D) Increase the concentration of I2.
E) Make the Cu electrode smaller.
Correct Answer:

Verified
Correct Answer:
Verified
Q66: You determine that for proper protection of
Q67: How is aluminium protected from oxidation?(If needed,
Q68: Consider the redox reaction of permanganate
Q69: For the galvanic cell shown in the
Q70: Draw three molecular pictures illustrating direct electron
Q72: Use the half-reaction method to balance
Q73: Aluminium is used in a battery
Q74: Calculate the equilibrium constant for the
Q75: For the following galvanic cell what will
Q76: Which of the following is NOT