Multiple Choice
For the following galvanic cell what will be its potential when the reaction reaches equilibrium?(If needed, refer to Table 17-1in the text ) 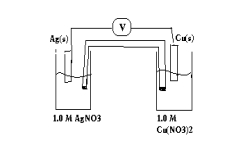
A) 0.0 V
B) 0.458 V
C) 1.142 V
D) 0.272 V
E) 1.26 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q66: You determine that for proper protection of
Q67: How is aluminium protected from oxidation?(If needed,
Q68: Consider the redox reaction of permanganate
Q69: For the galvanic cell shown in the
Q70: Draw three molecular pictures illustrating direct electron
Q71: For the working galvanic cell shown at
Q72: Use the half-reaction method to balance
Q73: Aluminium is used in a battery
Q74: Calculate the equilibrium constant for the
Q76: Which of the following is NOT