Multiple Choice
Consider the aqueous phase reaction between hydrogen gas and liquid bromine:H2(g) + Br2(g) 2HBr(g) Which of the following expressions accurately express the rate of the above reaction?
I. Reaction rate = 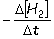
II. Reaction rate = 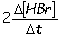
III. Reaction rate = 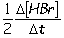
IV. Reaction rate = 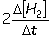
A) I and III
B) I and II
C) II and IV
D) III and IV
E) I only
Correct Answer:

Verified
Correct Answer:
Verified
Q12: The industrial production of 2-propanol involves the
Q13: The reaction of ozone with oxygen atoms
Q14: Consider the aqueous phase reaction between
Q15: Determine the rate law, given the mechanism
Q16: Assume that the following first-order reaction
Q18: The rate constant of the reaction,
Q19: The following rate constants were obtained
Q20: It has been suggested that the
Q21: NO<sub>2</sub> decomposes to form NO and O<sub>2</sub>.
Q22: Nitrogen dioxide molecules undergo oxygen exchange with