Essay
The industrial production of 2-propanol involves the reaction of propene with sulphuric acid and then water. Write the second step of the mechanism if the following is the first step: 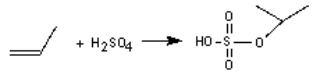
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: The following reaction takes place at
Q8: Trioxane undergoes decomposition to formaldehyde at
Q9: You are running late for a basketball
Q10: The following are initial rate data
Q11: Butadiene reacts to form its dimmer according
Q13: The reaction of ozone with oxygen atoms
Q14: Consider the aqueous phase reaction between
Q15: Determine the rate law, given the mechanism
Q16: Assume that the following first-order reaction
Q17: Consider the aqueous phase reaction between