Multiple Choice
Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the rate of increase of Cr3+ concentration expressed in terms of changing H3O+ concentration?
A) 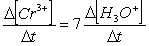
B) 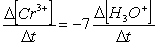
C) 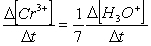
D) 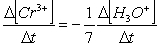
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: The saturation behaviour of enzyme systems (E
Q41: Why does the rate of a reaction
Q42: For a large number of reactions in
Q43: The reaction of NO<sub>2</sub> with CO to
Q44: Which of the following are bimolecular
Q46: The reaction of NO with O<sub>2</sub> to
Q47: It is determined that the charcoal in
Q48: At moderate temperatures, the rate law for
Q49: The rate law for the reaction of
Q50: The reaction of NO<sub>2</sub> with CO