The Following Reaction Takes Place at 80 Ru(NH3)5(H2O)3+ (Aq) + Cl- (Aq)
the Following Time and Concentration
Multiple Choice
The following reaction takes place at 80.1°C:
Ru(NH3) 5Cl2+ (aq) + H2O (l) Ru(NH3) 5(H2O) 3+ (aq) + Cl- (aq)
The following time and concentration data are collected: 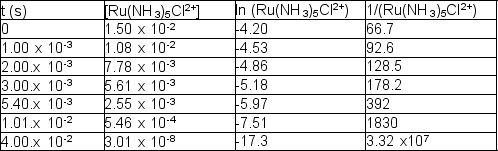 Which of the following is the correct value of the rate constant?
Which of the following is the correct value of the rate constant?
A) 3.28 1/M•s
B) 4.19 1/s
C) 419 1/s
D) 328 1/M•s
E) 328 1/s
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The reaction of ozone with oxygen atoms
Q3: Determine rate laws from concentration versus time
Q4: A proposed mechanism for the following
Q5: Nitrous oxide, N<sub>2</sub>O, decomposes on metal
Q6: The rate of a reaction<br>A) increases with
Q8: Trioxane undergoes decomposition to formaldehyde at
Q9: You are running late for a basketball
Q10: The following are initial rate data
Q11: Butadiene reacts to form its dimmer according
Q12: The industrial production of 2-propanol involves the