Short Answer
Sucrose, cane sugar, reacts with water in acid solution to give glucose and fructose, which have the same chemical formula.
C12H22O11 (aq) + H2O (l) 2 C6H12O6 (aq)
The following data were obtained at room temperature for sucrose: 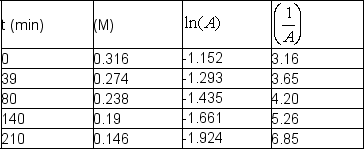 Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
Correct Answer:

Verified
first order; rate = ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q47: It is determined that the charcoal in
Q48: At moderate temperatures, the rate law for
Q49: The rate law for the reaction of
Q50: The reaction of NO<sub>2</sub> with CO
Q51: Cyclohexane is manufactured from the reaction
Q53: Which of the following does NOT occur
Q54: The reaction A + 2B
Q55: Nitrogen dioxide, NO<sub>2</sub> will react with
Q56: A 1.66 x 10<sup>-4</sup> mole sample of
Q57: Hydrogen and iodine react to form