Multiple Choice
Object A, with heat capacity CA and initially at temperature TA, is placed in thermal contact with object B, with heat capacity CB and initially at temperature TB. The combination is thermally isolated. If the heat capacities are independent of the temperature and no phase changes occur, the final temperature of both objects is:
A) (CATA - CBTB) /(CA + CB)
B) (CATA + CBTB) /(CA + CB)
C) (CATA - CBTB) /(CA - CB)
D) (CA - CB) 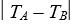
E) (CA + CB) 11ef181b_aacf_e68f_b5ce_19b6b0f164d3_TB10137_11
Correct Answer:

Verified
Correct Answer:
Verified
Q3: An incompressible liquid flows along the pipe
Q4: Two notes are an "octave" apart.The ratio
Q5: The mathematical forms for the three sinusoidal
Q6: The density of water is 1.0 g/cm<sup>3</sup>.
Q7: When the temperature of a copper
Q8: Which of the following represents a standing
Q9: Five hoops are each pivoted at a
Q10: If the length of a simple pendulum
Q11: A water line enters a house 2.0
Q12: The Doppler shift formula for the frequency