Multiple Choice

Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom shown in bold in the following compound: 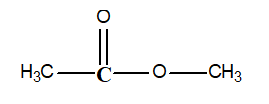
A) -1
B) 0
C) +1
D) +4
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Use a table of standard reduction potentials
Q19: refer to the following reaction which
Q20: Write a balanced chemical equation for
Q21: Which statement correctly describes the following
Q22: The potential of a cell at standard
Q24: How much Cl<sub>2</sub> gas would be collected
Q25: Which of the following isn't an
Q26: Which statement correctly describes the following
Q27: The half-reaction reduction potentials for the mercury(I)
Q28: Use a table of standard reduction potentials