Multiple Choice
Which of the following isn't an example of an oxidation-reduction reaction?
A) H2(g) + Cl2(g) 2 HCl(g)
B) Ag+(aq) + 2 NH3(g) 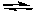 Ag(NH3) 2+(aq)
Ag(NH3) 2+(aq)
C) Hg2Cl2(s) + NH3(g) Hg(s) + HgNH2Cl(s)
D) 2 Mg(s) + O2(g) 2MgO(s)
E) Cl2(g) + 2 Br-(aq) 2 Cl-(aq) + Br2(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Write a balanced chemical equation for
Q21: Which statement correctly describes the following
Q22: The potential of a cell at standard
Q23: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q24: How much Cl<sub>2</sub> gas would be collected
Q26: Which statement correctly describes the following
Q27: The half-reaction reduction potentials for the mercury(I)
Q28: Use a table of standard reduction potentials
Q29: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q30: Suppose a beer can weighs 40.0 g.