Multiple Choice
Use a table of standard reduction potentials to determine which of the following statements is true for the electrochemical cell diagrammed below. 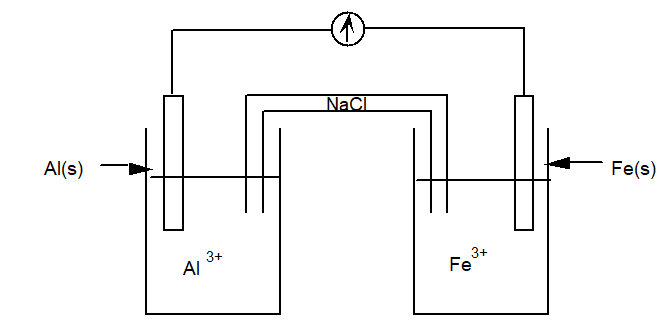
A) The Al is the cathode.
B) Electrons move from the Al electrode to the Fe electrode.
C) The mass of the Al electrode decreases.
D) Both (b) and (c) are true. .
E) Both (a) and (c) are true
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Which of the following isn't an
Q6: An electric current is passed through a
Q7: Calculate the complex dissociation equilibrium constant (K<sub>d</sub>)
Q8: A molten sample of TiCl<sub>4</sub> was electrolyzed
Q9: Which of the following is the
Q11: Use the following half reactions and accompanying
Q12: What is the ratio by weight of
Q13: Calculate the weight of sodium metal that
Q14: Calculate the amount of aluminum produced in
Q15: Which of the following electrolysis processes will