Multiple Choice
Calculate the complex dissociation equilibrium constant (Kd) for the Cd(NH3) 42+ complex from the following data at 298K. 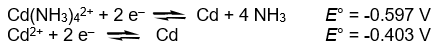
A) 2.7 x 10-7
B) 0.19
C) 6.6
D) 3.6 x 106
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q3: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q4: Cobalt(III) oxide reacts with hydrogen gas
Q5: Which of the following isn't an
Q6: An electric current is passed through a
Q8: A molten sample of TiCl<sub>4</sub> was electrolyzed
Q9: Which of the following is the
Q10: Use a table of standard reduction potentials
Q11: Use the following half reactions and accompanying
Q12: What is the ratio by weight of