Multiple Choice
Calculate the value of the Ksp for CdS at 25 °C from the following data.
CdS + 2 e- 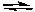 Cd + S2- E° = -1.21 V
Cd + S2- E° = -1.21 V
Cd2+ + 2 e- 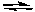 Cd E° = -0.40 V
Cd E° = -0.40 V
A) 3 x 10-55
B) 4 x 10-28
C) 2 x 10-14
D) 3 x 1027
E) 6 x 10
Correct Answer:

Verified
Correct Answer:
Verified
Q36: A current of 10.0 amps over a
Q37: refer to the following reaction in
Q38: For the reaction:<br>3 Sn<sup>2+</sup>(aq) + Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup>(aq)
Q39: Which of the following reagents should react
Q40: Which of the following isn't true?<br>A) The
Q42: There are two half-reactions for the reduction
Q43: refer to the following incomplete, unbalanced equation<br>Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup>(aq)
Q44: Redox Reactions in Basic Solutions<br>refer to
Q45: Given the following half-cell reduction potentials,
Q46: The equilibrium constant at 298 K