Multiple Choice
refer to the following reaction in acid solution.
CuS(s) + NO3-(aq) 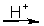 Cu2+(aq) + SO42-(aq) + NO(g)
Cu2+(aq) + SO42-(aq) + NO(g)
-In the balanced half-reaction for the NO3- ion, how many electrons are involved?
A) 1
B) 2
C) 3
D) 4
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q32: A voltaic cell is constructed with
Q33: Use the table of electrode potentials
Q34: What will be the coefficients of
Q35: Balance the following oxidation-reduction equation.<br>HI(aq) +
Q36: A current of 10.0 amps over a
Q38: For the reaction:<br>3 Sn<sup>2+</sup>(aq) + Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup>(aq)
Q39: Which of the following reagents should react
Q40: Which of the following isn't true?<br>A) The
Q41: Calculate the value of the K<sub>sp</sub> for
Q42: There are two half-reactions for the reduction