Multiple Choice
Consider the following reaction:
H2PO4-(aq) + HCO3-(aq) 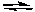 H2CO3(aq) + HPO42-(aq)
H2CO3(aq) + HPO42-(aq)
Brønsted would identify the acidic species as:
A) H2PO4- and H2CO3
B) H2PO4- and HPO42-
C) HCO3- and H2CO3
D) HCO3- and HPO42-
E) H2CO3 and HPO42-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Which of the following would be the
Q19: A 0.10 M solution of a weak
Q20: KHS can act as a weak acid
Q21: Which of the following solutions would have
Q22: The hydride ion, (H<sup>-</sup>), is a stronger
Q24: What would happen if more formic acid
Q25: Which of the following groups contains salts
Q26: A solution of weak base is added
Q27: Which of the following is correct (assume
Q28: Use the following information to answer <br>H<sub>2</sub>Se: