Multiple Choice
KHS can act as a weak acid or a weak base.
HS-(aq) + H2O(l) 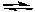 S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
HS-(aq) + H2O(l) 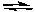 H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
Which of the following statements is correct?
A) A solution of 0.1 M K2S and 0.1 M KHS will be acidic.
B) A solution of 0.01 M H2S will have a pH = 2.
C) A solution of 0.1 M KHS will be weakly basic.
D) H2S is over 90% ionized in water solution.
E) The S2- ion concentration in a 0.1 M KHS solution does not depend on pH.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What can be correctly concluded from the
Q16: Which of the following would be the
Q17: What is the H<sub>3</sub>O<sup>+</sup> ion concentration in
Q18: Which of the following would be the
Q19: A 0.10 M solution of a weak
Q21: Which of the following solutions would have
Q22: The hydride ion, (H<sup>-</sup>), is a stronger
Q23: Consider the following reaction:<br>H<sub>2</sub>PO<sub>4</sub><sup>-</sup>(aq) + HCO<sub>3</sub><sup>-</sup>(aq) <img
Q24: What would happen if more formic acid
Q25: Which of the following groups contains salts