Multiple Choice
What can be correctly concluded from the fact that the following acid-base reaction proceeds to the right, as written?
NH2-(aq) + HSO4-(aq) 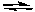 NH3(aq) + SO42-(aq)
NH3(aq) + SO42-(aq)
A) NH3 is a stronger base than NH2-.
B) NH3 is a stronger acid than HSO4-.
C) NH3 is a weaker acid than NH2-.
D) NH3 is a weaker base than HSO4-.
E) NH3 is a weaker acid than HSO4-.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Using data from the previous problem, what
Q11: Which of the following statements is true?<br>A)
Q12: If the strength of the following acids
Q13: When NH<sub>4</sub>Cl is added to a 0.10
Q14: What is the [HOAc]/[OAc<sup>-</sup>] ratio in an
Q16: Which of the following would be the
Q17: What is the H<sub>3</sub>O<sup>+</sup> ion concentration in
Q18: Which of the following would be the
Q19: A 0.10 M solution of a weak
Q20: KHS can act as a weak acid