Multiple Choice
Of the following, which is the strongest acid (lone pairs omitted for clarity) ?
A) 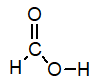
B) 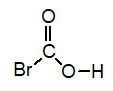
C) 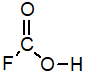
D) 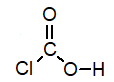
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q104: Which of the following solutions will show
Q105: Which compound cannot act as a Brønsted
Q106: Sodium fluoride, NaF (a soluble salt), is
Q107: What is the value of K<sub>b</sub> for
Q108: K<sub>b</sub> for methylamine (CH<sub>3</sub>NH<sub>2</sub>) is the equilibrium
Q110: Which of the following compounds would give
Q111: Which of the following structural factors is
Q112: What is the pOH of a 0.025M
Q113: Which of the following isn't an acid-base
Q114: There are many ways of "fluoridating" water.