Multiple Choice
Which of the following isn't an acid-base reaction?
A) 2 HgCl2(s) + 2 CH3Li(l) 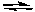 Hg(CH3) 2(l) + 2 LiCl2(s)
Hg(CH3) 2(l) + 2 LiCl2(s)
B) 2 NaCl(s) + H2SO4(aq) 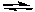 Na2SO4(aq) + 2 HCl(g)
Na2SO4(aq) + 2 HCl(g)
C) Na2CO3(aq) + H2SO4(aq) 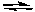 Na2SO4(aq) + 2 H2O(l) + CO2(g)
Na2SO4(aq) + 2 H2O(l) + CO2(g)
D) H2C2O4(aq) + 2 NaOH(aq) 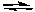 Na2C2O4(aq) + 2 H2O(l)
Na2C2O4(aq) + 2 H2O(l)
E) All of the above are acid-base reactions.
Correct Answer:

Verified
Correct Answer:
Verified
Q108: K<sub>b</sub> for methylamine (CH<sub>3</sub>NH<sub>2</sub>) is the equilibrium
Q109: Of the following, which is the strongest
Q110: Which of the following compounds would give
Q111: Which of the following structural factors is
Q112: What is the pOH of a 0.025M
Q114: There are many ways of "fluoridating" water.
Q115: The compound HB can be represented as
Q116: Which of the following statements is true?<br>A)
Q117: Rank the following in order of increasing
Q118: Which of the following is the weakest