Multiple Choice
Ammonia and the ammonium ion form a conjugate acid-base pair.
NH3(aq) + H2O(l) 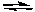 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10.
A) 4.7
B) 5.1
C) 5.7
D) 8.9
E) 11.1
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Calculate the pH of a buffer prepared
Q35: At what point in the following titration
Q36: Calculate the approximate pH of a 0.100
Q37: Which of the following equations is valid
Q38: What is the H<sub>3</sub>O<sup>+</sup> ion concentration in
Q40: Use the following acid-dissociation equilibrium constants for
Q41: The dissociation of water into H<sub>3</sub>O<sup>+</sup> and
Q42: Which of the following acid-base reactions should
Q43: Sulfurous acid (H<sub>2</sub>SO<sub>3</sub>) is an acid and
Q44: When NH<sub>4</sub>Cl is dissolved in water the