Multiple Choice
At what point in the following titration curve would the pH of the solution be equal to the pKa of the acid?
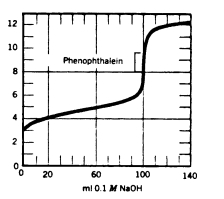
A) 0 mL
B) 50 mL
C) 95 mL
D) 100 mL
E) 120 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Which of the following isn't a Brønsted
Q31: The addition of sodium formate (HCO<sub>2</sub>Na) to
Q32: Calculate the pH of a solution prepared
Q33: What is the conjugate base of HSO<sub>4</sub><sup>-</sup>?<br>A)
Q34: Calculate the pH of a buffer prepared
Q36: Calculate the approximate pH of a 0.100
Q37: Which of the following equations is valid
Q38: What is the H<sub>3</sub>O<sup>+</sup> ion concentration in
Q39: Ammonia and the ammonium ion form a
Q40: Use the following acid-dissociation equilibrium constants for