Multiple Choice
Use the following graph for answering
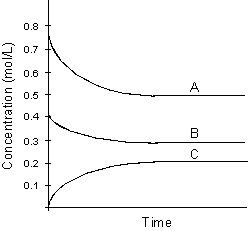
-Which of the following will be true of the equilibrium constant, Kc, for the reaction described in the above graph?
A) Kc 1
B) Kc 0
C) Kc < 1
D) Kc > 1
E) cannot be determined from graph
Correct Answer:

Verified
Correct Answer:
Verified
Q84: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q85: Use the following graph for answering <br>
Q86: If 1.3 x 10 <sup>-</sup> <sup>5</sup> moles
Q87: For the reaction below, 0.0500 moles of
Q88: The following chemical reaction has reached
Q89: For the reaction:<br>CO(g) + Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q91: Calculate the NO, NO<sub>2</sub>, and O<sub>2</sub> concentrations
Q92: What would happen if F<sub>2</sub> was removed
Q93: Calculate the equilibrium concentrations of SO<sub>3</sub>, SO<sub>2</sub>,
Q94: For the following reaction<br>2 C<sub>2</sub>H<sub>6</sub>(g) +