Multiple Choice
What would happen if F2 was removed from the following reaction while it was at equilibrium?
Cl2(g) + 3 F2(g) 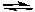 2 ClF3(g)
2 ClF3(g)
A) The concentration of Cl2 would increase as the reaction returned to equilibrium.
B) The concentration of both Cl2 and F2 would increase as the reaction returned to equilibrium.
C) The concentration of Cl2 would decrease as the reaction returned to equilibrium.
D) The concentration of both Cl2 and F2 would decrease as the reaction returned to equilibrium.
E) The concentration of both F2 and Cl2 would remain the same because the reaction would be at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q84: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q85: Use the following graph for answering <br>
Q86: If 1.3 x 10 <sup>-</sup> <sup>5</sup> moles
Q87: For the reaction below, 0.0500 moles of
Q88: The following chemical reaction has reached
Q89: For the reaction:<br>CO(g) + Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q90: Use the following graph for answering
Q91: Calculate the NO, NO<sub>2</sub>, and O<sub>2</sub> concentrations
Q93: Calculate the equilibrium concentrations of SO<sub>3</sub>, SO<sub>2</sub>,
Q94: For the following reaction<br>2 C<sub>2</sub>H<sub>6</sub>(g) +