Essay
Assume an equilibrium constant Kc = 1.7 x 10-2 for the reaction:
N2(g) + 3 H2(g) 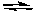 2 NH3(g)
2 NH3(g)
Calculate (to a first approximation) the equilibrium concentrations of NH3, H2 and N2 when 0.10 moles per liter each of N2 and H2 are mixed and allowed to react to equilibrium.
Correct Answer:

Verified
NH3 = 1.3...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q69: What is the concentration of Ag<sup>+</sup> (mol/L)
Q70: Which of the following equations describes
Q71: Calculate the concentrations of all reactants and
Q72: The following reactions are at equilibrium. Beside
Q73: What is the solubility in moles/L of
Q75: If the K<sub>sp</sub> of PbBr<sub>2</sub> is 8.9
Q76: What is the correct solubility constant expression
Q77: What is the correct solubility constant expression
Q78: For the reaction shown below, at equilibrium<br>I<sub>2</sub>(g)
Q79: What is the effect of increasing the