Multiple Choice
For the reaction shown below, at equilibrium
I2(g) + Br2(g) 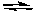 2IBr(g) Kc = 1.1 x 102
2IBr(g) Kc = 1.1 x 102
A) Reactants and products will be present in approximately equal concentrations.
B) Reactants will be favored.
C) Products will be favored.
D) Insufficient information is given to decide.
E) It is impossible for the reaction to reach equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q73: What is the solubility in moles/L of
Q74: Assume an equilibrium constant K<sub>c</sub> = 1.7
Q75: If the K<sub>sp</sub> of PbBr<sub>2</sub> is 8.9
Q76: What is the correct solubility constant expression
Q77: What is the correct solubility constant expression
Q79: What is the effect of increasing the
Q80: The following reactions are at equilibrium. Which
Q81: What is the K<sub>sp</sub> of SrF<sub>2</sub>(s) if
Q82: The following reaction is at equilibrium. Which
Q83: The following reaction was carried out at