Multiple Choice
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-A piece of copper metal weighing 145 grams was heated to 100°C and then dropped into 250 grams of water at 25°C. The copper metal cooled down and the water became warmer until both were at a temperature of 28.8°C. Calculate the amount of heat absorbed by the water. Assuming that the heat lost by the copper was absorbed by the water, what is the molar heat capacity of copper metal?
(CH2O = 75.376 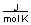 )
)
A) between 0 and 10 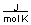
B) between 10 and 20 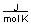
C) between 20 and 30 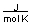
D) between 30 and 40 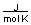
E) more than 40 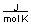
Correct Answer:

Verified
Correct Answer:
Verified
Q57: The following questions often assume that a
Q58: Arrange the following in order of increasing
Q59: Which of the following reactions is
Q60: Given the following data<br>3 H<sub>2</sub>(g) +
Q61: What is <span class="ql-formula"
Q63: What is the absolute value of the
Q64: Retailers purchase gasoline by weight so they
Q65: The reaction below shows two isomers of
Q66: Calculate the heat of combustion of
Q67: Calculate the heat required to transform