Multiple Choice
The reaction below shows two isomers of benzene, a compound with the formula C6H6. The structure of benzene was a point of great controversy in the late 1800's and the two structures shown in the reaction were the chief contenders.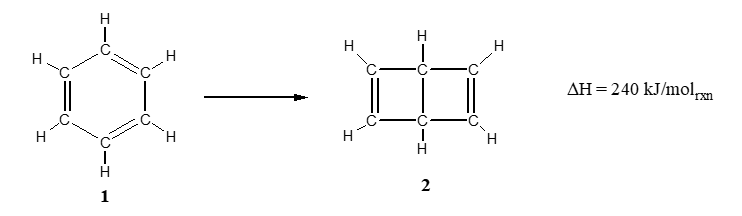
Which of the following statements is correct?
A) Isomer 1 is more stable than isomer 2
B) Isomer 2 is more stable than isomer 1
C) Isomer 2 has overall stronger bonds than isomer 1
D) Isomer 2 has stronger bonds and is more stable that isomer 1
E) Isomer 1 has weaker bonds and is more stable than isomer 2
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Which of the following reactions is
Q60: Given the following data<br>3 H<sub>2</sub>(g) +
Q61: What is <span class="ql-formula"
Q62: The following questions often assume that a
Q63: What is the absolute value of the
Q64: Retailers purchase gasoline by weight so they
Q66: Calculate the heat of combustion of
Q67: Calculate the heat required to transform
Q68: Calculate the average Si-Br bond strength
Q69: Predict which of the following gases has