Multiple Choice
What is the sign of the enthalpy of reaction for the reaction:
P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?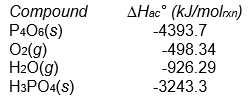
A) positive
B) negative
C) impossible to determine from the data
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Use the following data<br>2 H<sub>2</sub>(g) +
Q2: Calculate <span class="ql-formula" data-value="\Delta"><span
Q3: Use the following standard enthalpies of
Q4: Which of the following is a
Q6: (Note that some of these
Q7: Use the following standard enthalpies of
Q8: Which of the following reactions is
Q9: When carbon is burned in air
Q10: Calculate <span class="ql-formula" data-value="\Delta"><span
Q11: (Note that some of these are