Multiple Choice
Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the bond energy of N 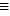 N?
N?
A) 237 kJ/mol
B) 158 kJ/mol
C) 473 kJ/mol
D) 946 kJ/mol
E) 1892 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Calculate <span class="ql-formula" data-value="\Delta"><span
Q3: Use the following standard enthalpies of
Q4: Which of the following is a
Q5: What is the sign of the
Q6: (Note that some of these
Q8: Which of the following reactions is
Q9: When carbon is burned in air
Q10: Calculate <span class="ql-formula" data-value="\Delta"><span
Q11: (Note that some of these are
Q12: Use the following standard enthalpies of