Multiple Choice
The bond-type triangle can be used for
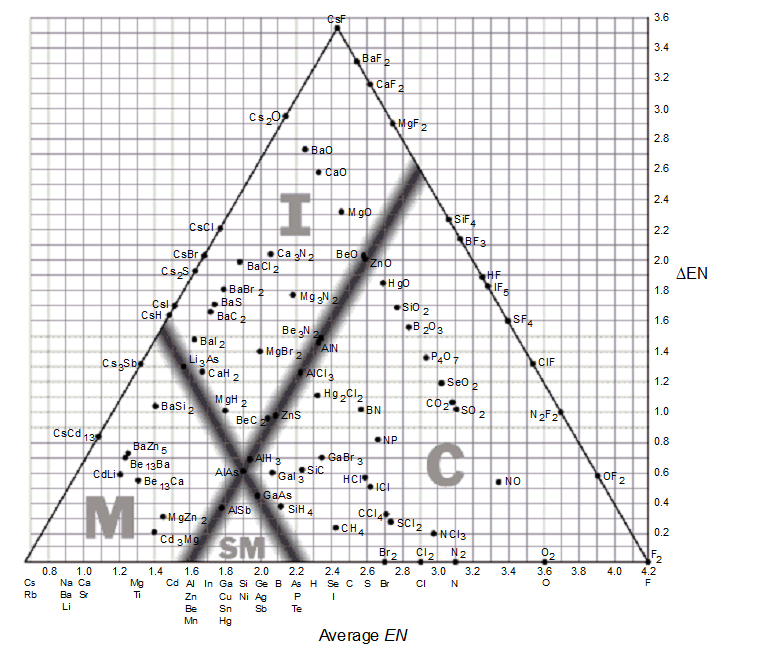
-Use a bond-type triangle to predict the correct arrangement of the following compounds in order of increasing covalent character.
Mg Zn2, CCl4, SiH4
A) Mg Zn2 < CCl4 < SiH4
B) CCl4 < Mg Zn2 < SiH4
C) CCl4 < SiH4 < Mg Zn2
D) MgZn2 < SiH4 < CCl4
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Which element is hydrogen most like to
Q26: What is the most likely electronic configuration
Q27: Determine the oxidation number of sulfur in
Q28: Identify the reducing agent and oxidizing
Q29: A mineral called montmorillonite will catalyze some
Q31: Give the chemical formula for hyposulfurous acid.<br>A)
Q32: Determine the oxidation number of the central
Q33: The bond-type triangle can be used for
Q34: In the following redox reaction which
Q35: Predict the formulas for neutral compounds containing