Multiple Choice
Determine the oxidation number of the central carbon atom in the following compound.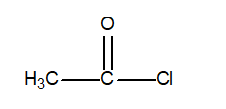
A) +2
B) -2
C) +4
D) -4
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Determine the oxidation number of sulfur in
Q28: Identify the reducing agent and oxidizing
Q29: A mineral called montmorillonite will catalyze some
Q30: The bond-type triangle can be used for
Q31: Give the chemical formula for hyposulfurous acid.<br>A)
Q33: The bond-type triangle can be used for
Q34: In the following redox reaction which
Q35: Predict the formulas for neutral compounds containing
Q36: What group of metals react with sulfur
Q37: Which of the following metals would be