Multiple Choice
Which of the following selection rules for quantum numbers is incorrectly stated?
A) n is any integer greater than or equal to zero.
B) l is any integer between zero and n - 1.
C) ml is any integer between -/ and +/.
D) ms is either + 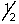 or -
or - 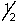 .
.
E) All of the above selection rules are correctly stated.
Correct Answer:

Verified
Correct Answer:
Verified
Q70: Which of the following atomic orbitals doesn't
Q71: In what group of the periodic table
Q72: A possible set of quantum numbers for
Q73: Which set of quantum numbers can be
Q74: Arrange the following atoms in order of
Q76: Give the full electron configuration of arsenic,
Q77: Why is the first ionization energy of
Q78: Which transition in the spectrum of the
Q79: In which of the following groups of
Q80: In period 6 of the periodic table,