Multiple Choice
A possible set of quantum numbers for the last electron added to form a gallium atom (Z = 31) in its lowest energy state is:
A) 3, 1, 0, - 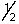
B) 3, 2, 1, 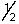
C) 4, 0, 0, 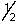
D) 4, 1, 1, 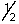
E) 4, 2, 2, 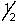
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: Which element is represented by the following
Q68: Which of the following transitions in the
Q69: Which ion, Na<sup>+ </sup>or K<sup>+</sup>, is the
Q70: Which of the following atomic orbitals doesn't
Q71: In what group of the periodic table
Q73: Which set of quantum numbers can be
Q74: Arrange the following atoms in order of
Q75: Which of the following selection rules for
Q76: Give the full electron configuration of arsenic,
Q77: Why is the first ionization energy of