Multiple Choice
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle.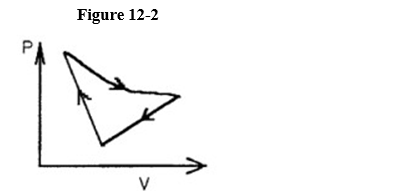
If the process was carried out in a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-2, then the change of internal energy over the full cycle
A) is positive.
B) is negative.
C) is zero.
D) cannot be determined from the information given.
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Is it possible for heat to travel
Q13: An ideal gas is compressed isothermally from
Q14: A coal-fired plant generates 600 MW of
Q15: Coefficient of performance is<br>A) Q<sub>out</sub>/W<sub>in</sub>.<br>B) Q -
Q16: Isobaric work is<br>A) p <span
Q18: The Otto cycle has how many strokes
Q19: The work done by the gas is
Q20: The Department of Energy develops a new
Q21: A gas is confined to a rigid
Q22: Referring to Figure 12-9, a substance carried